Haemochromatosis: More Common Than You Think! Let Us Learn about Its Origin and Pathophysiology.
Before we begin
The Haemochromatosis series will be a 2 part blog where the first blog shall be about Haemochromatosis with its origin, genetics and pathophysiology. The second part will be an interview of a patient living with Haemochromatosis, how they found out, their daily challenges, and how they cope with this condition every day.
Part 2 can be found here: “Haemochromatosis: Its Impact on People's Life and How They Cope with This Condition”.
Introduction
A) Armand Trousseau. Lithography by J.B.A. Lafosse from 1866 (CC BY 4.0). B) First page of article from 1865 in which Trousseau describes the first known case of Haemochromatosis. Clinique médicale de l’Hôtel-Dieu de Paris, digitised by the University of Ottawa. From: Tidsskrift Den Norske Legeforening website.
Haemochromatosis is described as a metabolic disorder and involves the abnormal absorption of iron in the body. The accumulation of iron in excess causes life-threatening symptoms and severe outcomes on the affected organs, especially the liver. The first description of Haemochromatosis was done in 1865 by Dr Trousseau in the French pathology literature (Bacon, 2012). Later in 1889, it was described by the German pathologist von Recklinghausen who first used the term Haemochromatosis meaning pigmentation/colour ‘chrom’ and ‘heam’ which is a prefix referring to the blood (Bacon, 2012). He saw the liver to be full of iron and bleeding. At that stage, the link between iron overload, diabetes and liver cirrhosis was unknown. By 1935, Dr Joseph Sheldon concluded, without the use of modern medicine, that Haemochromatosis was an inherited disorder which involved an excess iron deposition within the body (Bacon, 2012). In1976, Haemochromatosis is described as an autosomal recessive disorder, and the gene has been located on chromosome 6. By 1996, the HFE (High Fe2+/Iron, Homeostatic Iron Regulator) gene has been identified along the homozygosity for a single missense mutation (C282Y) of the HFE gene (Bacon, 2012). Throughout the birth of medicine and its techniques through the centuries, Haemochromatosis is an interesting condition as for once ‘bloodletting’ is actually the right method of treatment.
Haemochromatosis is more common than the general population thinks. The prevalence of Haemochromatosis is 1 in 300 in the United States or 1 in 200 if the person is from a Northern European descendent and 1 in 9 carry the gene! There is good news when someone is affected by Haemochromatosis. The prognosis has a positive outlook, and if the condition is detected early with the right treatments, a patient can have a relatively normal life expectancy. This needs to be discovered before any damage has occurred. That is not to say a patient will have no challenges in their lives and that the condition will not be felt. All in all, it is best to stay informed, educated and be aware of what people have to go through with Haemochromatosis.
What is Haemochromatosis
Haemochromatosis is under-diagnosed compare to its prevalence within the population. There are a few reasons for this, these are:
There are no distinct or specific symptoms,
The condition takes a while to cause significant damage,
Lack of awareness that someone has the condition if they feel healthy. People generally feel fine with an elevated iron blood level.
People without the condition absorb only 1-2 mg of iron per day at the small intestine, whilst people with Haemochromatosis can absorb between 4-5 mg of iron a day and store between 15-40 grams of iron! That is a lot of iron for a specie that does not have a physiological regulation excretion pathway; hence, an accumulation of iron will occur in specific areas and cause damage to the organs. The most affected organs are: liver, gallbladder, spleen, thyroid gland, pituitary gland, pancreas, gonads, skin, heart, bones and joints.
The liver is the most affected organ due to having a high blood flow (1.5 litres per minute) which is made worst by the blood coming back (through the portal circulation) from the small intestine where the iron is mainly absorbed. Hemochromatosis.org mentions that the unregulated amount of iron in the brain has been observed with patients who had epilepsy, multiple sclerosis, Huntington's disease, early-onset Parkinson's and Alzheimer's disease.
Iron and its regulation
Iron is extremely useful in the human body and vital for survival. It is involved in oxygen transport and cellular respiration as iron acts as an electron donor and acceptor. Unregulated level of iron can be lethal and toxic as the high amount of iron causes an overproduction of reactive oxygen species (ROS). ROS causes damage to proteins, DNA/RNA and lipids, and in theory, contribute to the physiology of ageing.
Distribution of iron in the adult human body and regulation of iron traffic. From: Sebastiani G et al.
A well-nourished person would have about 4-5 grams of iron in their body, where 2.5 grams is used for oxygen transport in the haemoglobin, and the rest is in ferritin. Ferritin keeps the iron in a safe and stable compound and is found mainly in the liver, spleen, and bone marrow. The liver stores of ferritin are the primary source of iron reserve for the body. About 400 mg of iron is utilised for cellular respiration around the body (for uses involving redox reactions), and about 3-4 mg circulates the plasma bound to transferrin. At all times, freely soluble iron is kept at a low level due to its toxicity.
Without going into too many details as it can get complicated and tedious, the homeostasis of iron is regulated in two different ways, where 1. the iron absorption is controlled by enterocytes (intestinal absorptive cells) through the nutrition of a person and 2. the uncontrollable loss of iron through cell loss, red blood cell (RBC) loss, sweating, urine (abnormal), faeces and injuries. A lot of recycling occurs within the body as the iron content in the body is highly conserved. Every day we need 20 mg of iron to create the RBC that are necessary for the body. The iron from the old RBC is thus recycled and conserved. Only a small quantity of iron is lost per day, where men lose about 1 mg of iron per day, and women would lose about 1.5-2 mg a day. The Institute of Medicine (US) Panel on Micronutrients mentions that “the Recommended Dietary Allowance (RDA) for all age groups of men and postmenopausal women is 8 mg/day” and that “the RDA for premenopausal women is 18 mg/day”. They also mention that the median dietary intake is 16 to 18 mg/day for men and 12 mg/day for women, and that an adult upper limit would be 45 mg/day (at that high amount gastrointestinal distress will occur). Out of this normal nutrition, a male adult only needs to absorb about 8-10%, which is equivalent to 1 mg/day to maintain the iron balance (IMPM, 2001). A menstruating woman should absorb 1.5 mg/day. However, due to menstrual variation, a woman adult may absorb up to 3.4 mg/day (IMPM, 2001). During pregnancy, especially towards the end, 4 to 5 mg/day would be necessary (IMPM, 2001). As mention before, a person with Haemochromatosis will absorb 4 times its equivalence, which is equivalent to 32-40% (4-5 mg/day). Vegetarians and vegans need to pay extra attention in making sure they eat iron-rich substitution. Blood donors are also at risk of having low iron, however the blood banks tests for haemoglobin in making sure they can donate.
The Institute of Medicine (US) Panel on Micronutrients has excellent and reliable content about iron and other dietary elements.
It’s common and it’s genetics
The general cause of Haemochromatosis is that there is too much iron absorption and deposition in specific organs. The body is overloaded with iron, and there is no specific excretory pathway for iron. Hence, once iron is absorbed, it stays. The outcome of untreated Haemochromatosis is organ failure and death.
Where does Haemochromatosis come from? People with Northern European of caucasian descendants are more at risk inheriting the defective genes. It was thought that Haemochromatosis came from the Celtic population in the middle ages and may have provided an advantage over people who did not have the genetic mutation. With a life expectancy of about 40 years and where iron deficiency was common, it makes sense that people with the mutated gene had a normal life and did not directly die from the condition. It is also believed that Haemochromatosis may have originated as far back as the Neanderthal era where they have been tested positive (through DNA testing) for diabetes, cancer and other diseases. Why not Haemochromatosis? However, a study has shown that they have found the Haemochromatosis C282Y allele in the Celtic population contributing to the Irish genome as far back as 4,000 years ago (Cassidy LM et al, 2016) and it is speculated that the “Viking migrations were largely responsible for the distribution of this mutation” (Distante S et al, 2004).
Statistics about haemochromatosis:
1 in 9 people are carriers of the defective gene and are most likely not aware of it.
1 in 200 to 300 has the condition. Depending on the heritage history as Northern Europeans (especially from Celtic backgrounds) will have a prevalence of 1:200. The prevalence can be up to 1:500.
85% ± 5% of Caucasians are diagnosed with homozygous Cys282Tyr (C282Y and C282Y). There is a range of 61-92% for other population around the world.
As an autosomal recessive condition, both genders will be affected by the inheritance of the hemochromatosis gene equally. Both genders demonstrated similar hepatic iron concentrations. However, the clinically significant iron overload will present itself in about 30% of C282Y homozygous males, 1% in the pre-menopausal stage in females and 14% post-menopausal females (Beaton MD, 2007; General Practice Supervisors Australia). Milman et al have shown that if men diagnosed with Haemochromatosis (C282Y homozygous), 38% will present with liver disease, rheumatoid arthritis, osteoarthritis or diabetes mellitus compared to 16% in men that do not have C282Y mutations. The same can be said in women, where 27% with Haemochromatosis versus 17% who do not have the mutations. What is crucial is that women must be checked as it is a myth that women do not get Haemochromatosis.
The most common compound heterozygotes is the C282Y/H63D combination.
People with homozygote (C282Y and C282Y) and the compound heterozygote (C282Y and H63D) are the most at risk.
Types 1, 2 and 3 Haemochromatosis is inherited in an autosomal recessive pattern. Both copies of the gene in each cell have mutations as each parent must have been carriers. Type 4 Haemochromatosis contains an autosomal dominant inheritance pattern. Therefore one copy of the altered gene in each cell is sufficient to cause the disorder. Do note that type 2 and 4 have subtypes. Type 2 to 4 are rare, and they all act on different genes, proteins and have different severity and disease onsets.
The Genetics
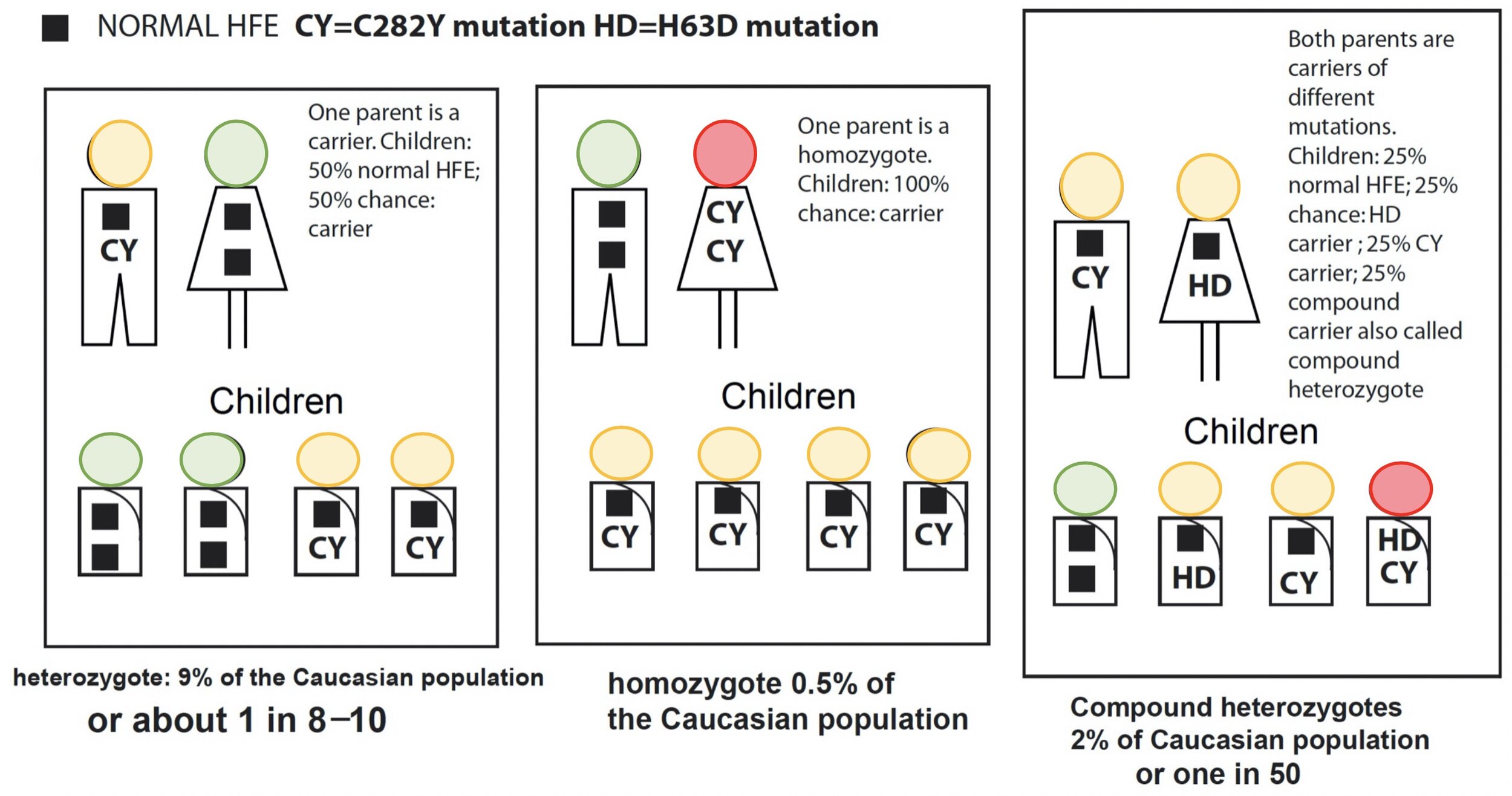
Everybody will have two copies of the gene HFE where one came from Dad and the other from Mom. Hereditary Haemochromatosis is described as an autosomal recessive condition, which means having a copy of the defective genes should not demonstrate any clinical symptoms. People carrying one defective gene are considered to be carriers. This is in contrast to autosomal dominant where one defective gene will cause clinical symptoms such as Huntington's disease; hence, the defective gene is dominating the normal HFE gene. Interestingly, it is possible to receive 2 different defective genes making the person a compound heterozygotes (e.g. C282Y and H63D). This person will demonstrate clinical symptoms. It is also possible to have both of the same defective genes (e.g. C282Y and C282Y), inherited from their parents who most likely were carriers and never knew their whole life. Figure 1 below demonstrates the chance of passing down the defective genes to their offsprings. Bear in mind that Haemochromatosis is an autosomal recessive condition meaning you need both defective genes from Mom and Dad.
Figure 1: Demonstrates the inheritance patterns of HFE mutations in families. From: Hemochromatosis.org.
Below is an explanation of Haemochromatosis by using the Punnett square technique to better visualise the autosomal recessive transmission.
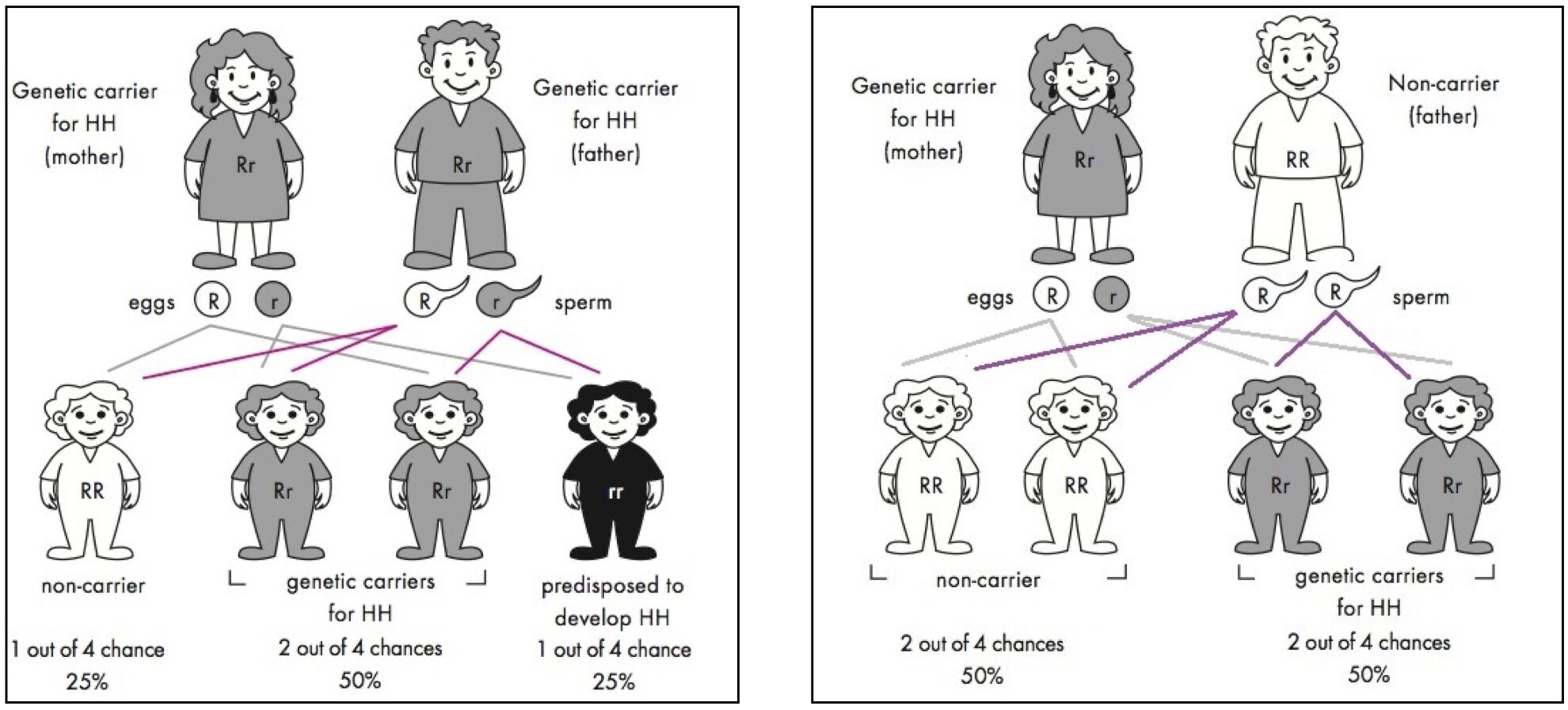
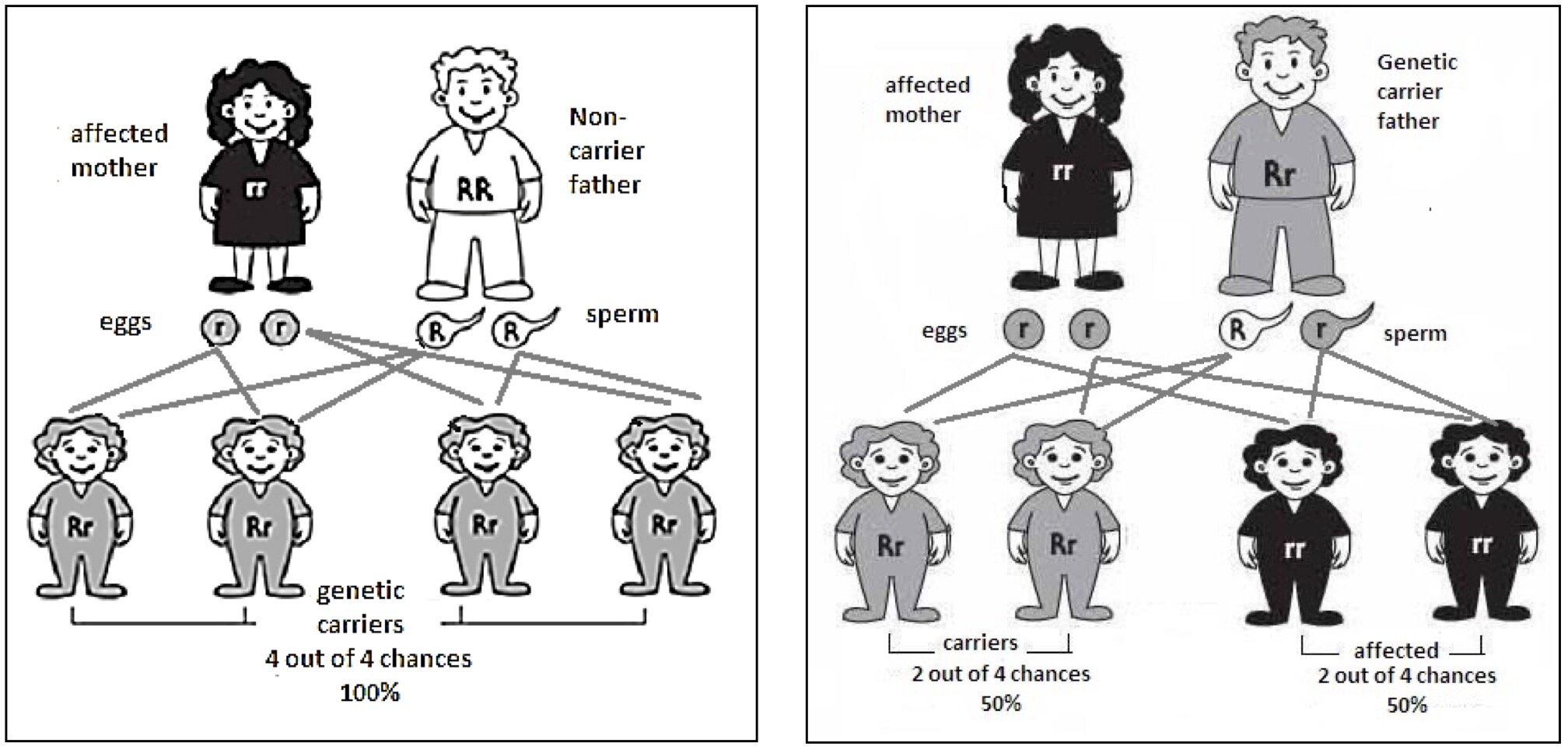
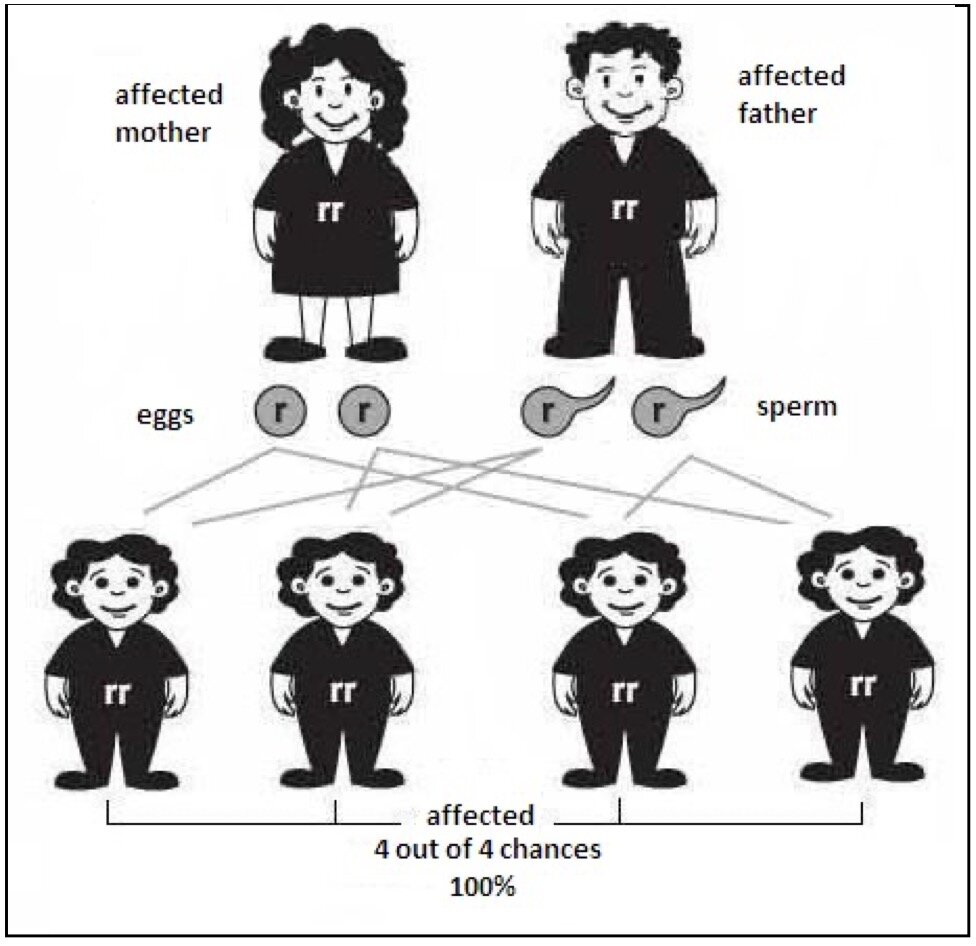
Figure 2: Demonstrates how changing a nucleotide can change the amino acid Lysine to be made.
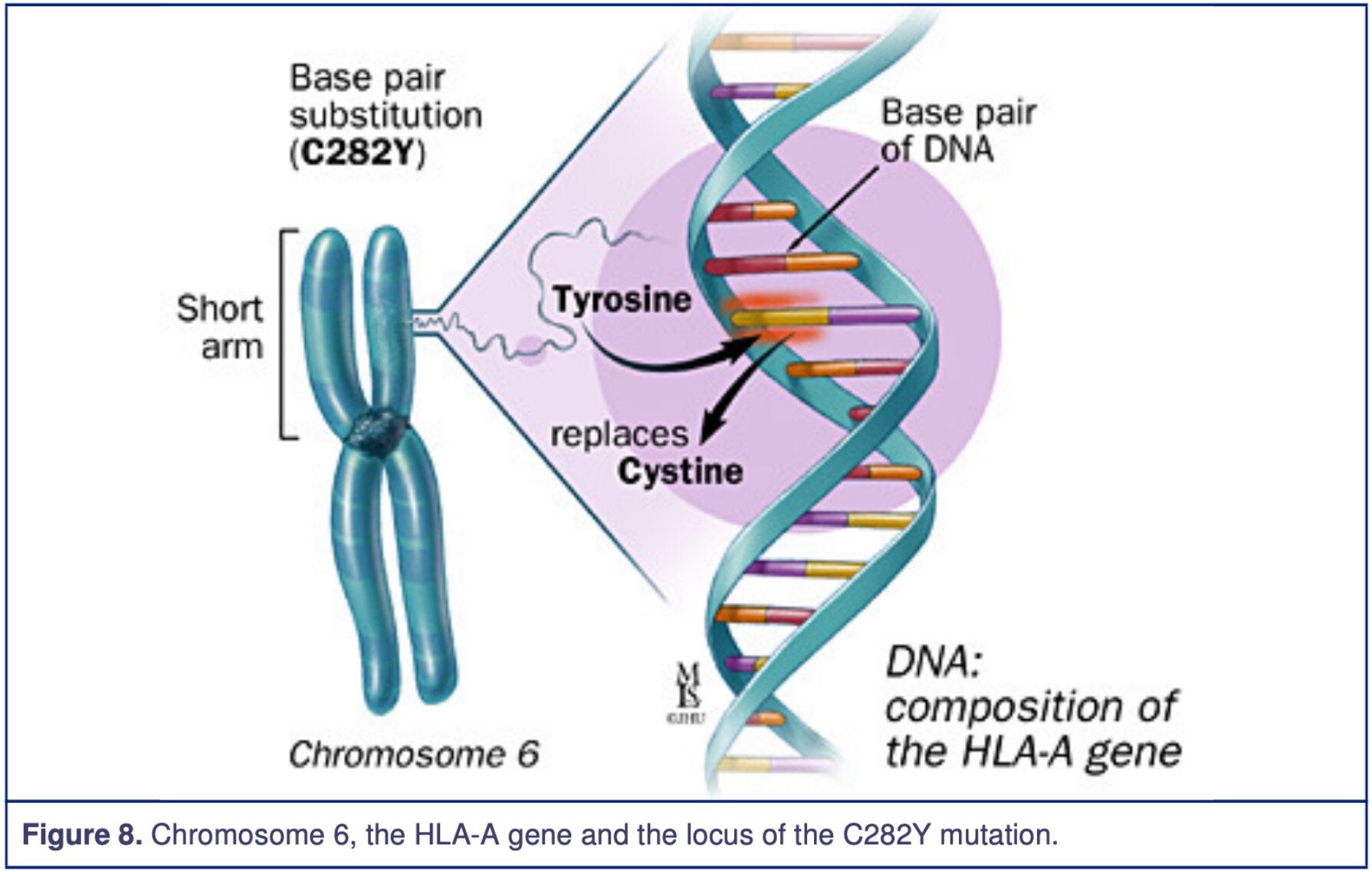
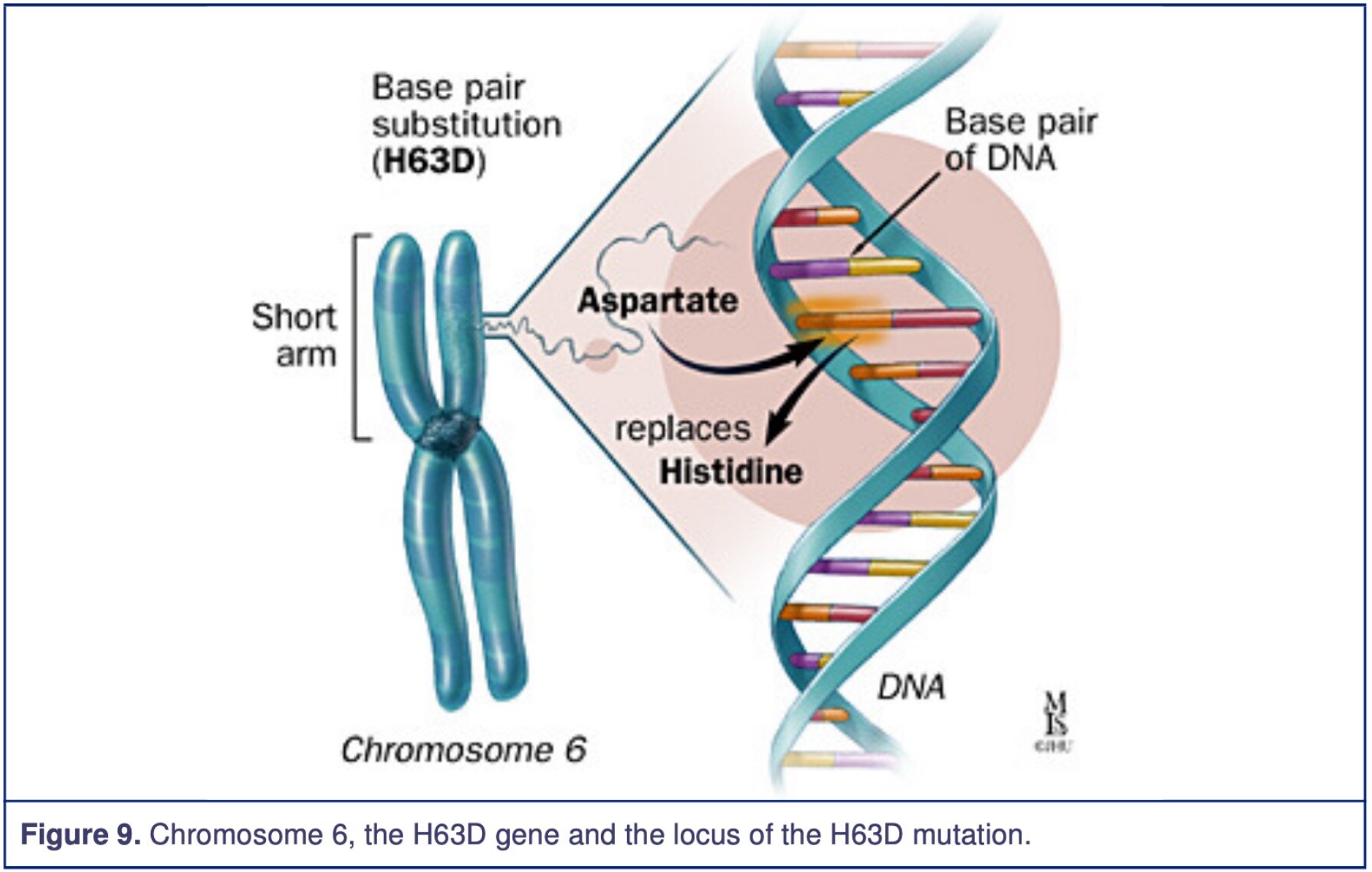
It has been identified that the mutated HFE gene (High Fe2+/Iron, Homeostatic Iron Regulator) is associated with the HLA-A gene, located on chromosome 6 short arm. The Haemochromatosis defective gene codes for a protein called HFE. This protein is involved in the iron absorption, which is part of the iron transport system at the duodenal mucosal cells. The protein modulates the uptake of transferrin-bound iron; however, the mutation causes up-regulation, which increases iron absorption. On the HFE gene at the nucleotide 845, there is a transition point mutation of the C282Y allele, meaning guanine was changed to adenosine. The result is a missense mutation of Cys282Tyr (Cysteine to Tyrosine) seen in figure 2, which demonstrates examples of how an amino acid can change. The amino acid number 282 has been substituted from Cysteine to Tyrosine, and the protein 3-dimensional structure has now been altered. Another identified and relevant mutation that has been found is His63Asp (Histidine to Aspartic Acid), meaning the amino acid number 63 has been changed from Histidine to Aspartic Acid resulting in the change of the protein 3-dimensional structure.
Abbreviations of amino acids with their name, three-letter code and one letter code:
Cysteine, Cys, C.
Tyrosine, Tyr, Y.
Histidine, His, H.
Aspartic Acid, Asp, D.
The figures below demonstrate the missense mutation on the HFE gene at the proximity of the HLA-A locus. The figures are from Johns Hopkins Medicine.
Genetic definitions
Gene: a specific sequence of nucleotides in DNA or RNA that is located usually on a chromosome and that is the functional unit of inheritance controlling the transmission and expression of one or more traits by specifying the structure of a particular polypeptide and especially a protein or controlling the function of other genetic material.
Allele: each of two or more alternative forms of a gene that arise by mutation and are found at the same place on a chromosome. An example is the colour of a flower.
Locus: the position of a gene or mutation on a chromosome.
Autosomal recessive: a genetic condition that appears only in individuals who have received two copies of an autosomal gene, one copy from each parent. The gene is on an autosome, a nonsex chromosome. The parents are carriers who have only one copy of the gene and do not exhibit the trait because the gene is recessive to its normal counterpart gene. A person with one defective gene is a carrier and has a 50% chance of passing their defect gene. If a person suffers from their autosomal recessive disease they have 100% chance of passing their defect genes to their offsprings, however, they will only be carriers as their partners will pass down their more dominant normal genes.
Autosomal dominant: a pattern of inheritance in which an affected individual has one copy of a mutant gene and one normal gene on a pair of autosomal chromosomes. One defect gene is enough to dominate and express the trait even if the other copy is normal. Individuals with autosomal dominant diseases have a 50-50 chance of passing the mutant gene to which the offspring will have the disease regardless.
Heterozygotes: an individual having two different alleles of a particular gene or genes, and so giving rise to varying offspring.
Homozygotes: an individual having two identical alleles of a particular gene or genes and so breeding true for the corresponding characteristic.
Compound heterozygotes: the presence of two different mutant alleles at a particular gene locus. In our Haemochromatosis case, it may as well be C282Y and H63D.
What are the symptoms
Usually, the symptoms start to appear at the age of 40 for men but at a later age for women. Some literature mention that men can start experiencing symptoms in their late 20s to early 30s. Women may experience symptoms 10-15 years later. This is due to their menstrual cycles where iron is lost due to loss of RBCs. Initially, people with Haemochromatosis will experience fatigue and joint pain which is difficult to diagnose due to age and many diseases showing these symptoms. Other common symptoms include:
Irregular heartbeat or heart flutter.
Lack of energy, lethargy/weakness.
Joint pain.
Memory fog or vertigo.
Loss of sex drive, impotence.
Abdominal pain.
Hair loss.
Skin pigmentation.
If left untreated, these complications will occur:
Liver: fibrosis, cirrhosis, cancer, enlarged liver, and liver failure.
Gallbladder disease.
Endocrine: hormones imbalances, diabetes mellitus, hypothyroidism, hypogonadism, infertility or impotence, pancreas disturbance or pancreatic cancer, pituitary disturbance.
Skin: abnormal colour such as bronze, reddish or ashen-grey.
Bone and joint: exacerbated arthritis, osteoarthritis or osteoporosis in knuckles, ankles, and hips. Increases the chance of fractures.
Heart: heart failure, irregular heartbeat, heart attack, cardiomyopathy and an enlarged heart.
Spleen: enlarged spleen.
Depression.
The main cause is due to the excess iron deposition and increases the ROS concentration to which it causes damage to the cells. However, the bronze skin colour is due to the increase in melanin deposition. The arthritis is caused by calcium pyrophosphate crystal accumulation. Initially, arthritis will involve the second and third metacarpophalangeal joints and thereafter, it might include other significant joints. The anterior pituitary gland is affected by the iron deposition and as a result, releases less follicle-stimulating hormone (FSH) and luteinizing hormone (LH) resulting in amenorrhea in females and impotence in male.
The figure below is from Johns Hopkins Medicine and I highly recommend reading the material.
Testing and Treatment
Testing
A patient history, physical examination, blood tests and genetic testing are often sufficient for the diagnosis of Haemochromatosis. A liver biopsy may also be done for the diagnosis of Haemochromatosis but generally helps in the prognostic of the condition.
For blood tests, there needs to be an Iron Panel Test. This includes: serum iron, serum ferritin and total iron-binding capacity. Serum iron measures iron levels in serum, which represents iron that is almost completely bound to transferrin. Transferrin are iron-binding blood plasma glycoproteins that control the level of free iron (Fe) in biological fluids. The serum ferritin test measures the body's ability to store iron. Total iron-binding capacity measures the blood's capacity to bind iron with transferrin. Transferrin-saturation can be calculated by using the equation: serum iron/total iron-binding capacity. If the results demonstrate a high serum iron, high serum ferritin and a high transferrin-saturation with normal haemoglobin, the tests would suggest hereditary Haemochromatosis. Some studies suggest that the mean corpuscular volume (MCV) above >96% was a possible marker for Haemochromatosis.
Liver biopsy: is an excellent standard to see the prognosis and the extent of liver damage as well as quantifying the iron deposition inside the liver. Liver function tests can also be done to see if there has been any damage.
Genetic tests can be done to confirm the condition and distinguish which type and which mutation is involved. It is good to inform inform all first-degree relatives and advice for testing.
Treatment
The main goal of treatment is to reduce the amount of iron in the body. The obvious option would be therapeutic blood removal or phlebotomy (blood donations) until the blood ferritin level range reaches between 50-150 ng/mL. A patient may have to do several phlebotomies depending on their iron status and initially may have to give blood up to 2 times a week for up to a month. It may take years for the iron stores to be at a reasonable range, but an early diagnosis can reduce the amount of phlebotomies from 30 sessions or less. Some patients with heart diseases may take longer as they cannot tolerate that amount of blood removal per session. Once a patient has reached their maintenance range, they can do a blood donation every 2-4 months for the rest of their lives. Men may do between 3-4 sessions a year and pre-menopausal women may only have 1-2 sessions a year; however, the frequency is related to the iron load, which is dependent on the HFE gene. For example, homozygous H63D, after de-ironing, might never do venesections again compared to someone who is homozygous C282Y might do every 3 months. It is important to note that gender does not define the amount of venesections. What makes it different is if a woman is pre or post-menopausal.
The blood removal therapy decreases the likelihood of getting cancer, extends the life expectancy and improve or alleviate the symptoms associated with the excess iron deposition. However, we do need to mention that phlebotomies do not restore everything. Only 25% of patients improve with endocrine and joint problems. The cirrhosis (damage to the liver) cannot be reversed.
Chelation medications such as deferoxamine are medications that remove the excess iron for people who cannot give blood. It is either injected into the body or given as pills. Its mode of action is to bind to the iron and is then expelled through the stool or urine.
A liver transplant can be done for extreme cases. Sadly, the median survival rate is 2.8 years and the longest is 5.5 years.
Yearly physical examination should be done with blood tests and biannual imaging to see any progression and results of treatments.
Lifestyle modification
John Hopkins and the Mayo Clinic both agree that whilst undergoing treatment it would be a good idea to:
Reduce the iron intake, especially from iron supplements and multivitamins containing iron.
Reduce vitamin C supplements as they increase iron absorption; however, there is no need to restrict vitamin C in your diet. Do not stop eating fruits and vegetables.
Reduce alcohol intake. Alcohol damages the liver and may worsen the liver condition. This is important in Haemochromatosis patients as their liver is already at risk. Vitamin C and alcohol are found to increase iron absorption.
Avoid eating raw fish and shellfish: Haemochromatosis patients are susceptible to infections as some of these products may contain harmful bacterias such as vibrio vulnificus infection which is the leading cause of death related to seafood consumption in the United States.
There is no need to need to reduce iron intake in a diet as the venesections are very effective. However, if a patient chose to do so, they may want to reduce the consumption of red meat to approximately 90-120 g/day (Haemochromatosis, 2007).
Haemochromatosis Australia encourage to eat a healthy and balanced diet and not to focus too much on what you eat; they also advise:
B12 and folate supplements are helpful.
Tannins from tea and coffee, calcium in dairy products, some fish, fruit and vegetables inhibit or slow the absorption of iron.
Iron fortification can sometimes be found in bread, breakfast cereal, energy drinks and bars. Hence, be sure to avoid and check what you buy.
Pharmacy-only iron supplements will contain much higher levels of elemental iron. These products should only be taken for low serum ferritin detected on a blood test after consultation with a GP.
So do not worry too much about the iron in your food. Focus on staying healthy and eat a nutritious, balanced diet.
Haemochromatosis myths, what is true?
Below are some common myths that people think about Haemochromatosis. Beaton MD and Adam PC published a paper named “The myths and realities of hemochromatosis” in the Canadian Journal of Gastroenterology, it is very interesting and a lot more detailed.
Hemochromatosis is rare
As seen earlier, it is not. It is found that 1 in 9 people are carriers of the defective gene and are most likely not aware of it, and 1 in 200 to 300 has the condition.
Women are not affected by haemochromatosis
The condition affects and is inherited by both genders. Both genders demonstrated similar hepatic iron concentrations. Woman menstruating at pre-menopausal stage does cause significant blood loss. However, the clinically significant iron overload will present itself in about 30% of C282Y homozygous males, 1% in the pre-menopausal stage in females and 14% post-menopausal females (Beaton MD, 2007; General Practice Supervisors Australia). What is crucial is that women must be checked as it is a myth that women do not get Haemochromatosis and is often overlooked by the medical staff.
Most haemochromatosis patients are alcoholics
Alcoholics do have elevated serum ferritin levels and do have an increase iron deposition in the liver. However, studies have demonstrated that there is no increase in prevalence of alcoholism with people who have Haemochromatosis.
Many haemochromatosis patients have chronic viral hepatitis
This is not true. There is no evidence of having a higher prevalence in hepatitis B virus and hepatitis C virus with people who have Haemochromatosis.
Diabetes is a cardinal feature of haemochromatosis
Compared to a control population, people who have Haemochromatosis (C282Y homozygotes) do not show an increase in the prevalence of diabetes. However, Milman et al did mention that men diagnosed with Haemochromatosis (C282Y homozygous), 38% will present with liver disease, rheumatoid arthritis, osteoarthritis or diabetes mellitus compared to 16% in men that do not have C282Y mutations. The same can be said in women, where 27% with Haemochromatosis versus 17%. Diabetes is complicated is most likely multifactorial where other aspects are affected.
Most haemochromatosis patients have elevated liver enzymes
This is very interesting as one would be thinking that a condition that damages the liver would have elevated liver enzymes. What is important to take note is that Haemochromatosis does not fall under inflammatory liver disease, but a routine liver enzyme test should still be done just to be on the safe side.
Most patients with an elevated serum ferritin level have haemochromatosis
Many conditions demonstrate an elevated serum ferritin level such as: chronic inflammatory conditions such as rheumatoid arthritis, heavy alcohol use, Hodgkin lymphoma, hyperthyroidism, leukaemia, liver disease, porphyria, et cetera.
An elevated HAEMOGLOBIN is common in hemochromatosis
This is not a reliable marker to test Haemochromatosis. Genetic testing is the best outcome to find out about Haemochromatosis.
Children of hemochromatosis patients are at the highest risk of disease
Haemochromatosis is described as an autosomal recessive disorder, hence you need both parents to have a defective gene to cause a child to have Haemochromatosis. If one parent is C282Y homozygotes and passes down the defective gene, the normal gene passed down from the other parent will be dominant.
Genetic testing for haemochromatosis is a research tool
Now widely available, these test are cheap and powerful in diagnosing Haemochromatosis.
Hemochromatosis patients should be on a low-iron diet
This is also not true. There is a need to reduce the iron intake from supplements and multivitamins containing iron but there is no need to need to reduce iron intake in a diet as the venesections are very effective. A reduction in vitamin C supplements, alcohol consumption and raw fish/shellfish is beneficial.
Hemochromatosis is a progressive disease
Interestingly it is not. Some patients have refused to do any venesections and have not deteriorated further. Venesections does help in preventing further liver fibrosis.
Published 30th May 2020. Last reviewed 1st December 2021.
Reference
Australian College of Rural and Remote Medicine (ACRRM) Authors. Diagnosis And Management Of Haemochromatosis. Australian College of Rural and Remote Medicine (ACRRM) website. https://mycollege.acrrm.org.au/search/find-online-learning/details?id=1030&title=Diagnosis+And+Management+Of+Haemochromatosis. Updated 2020. Accessed March 25, 2020.
Bacon BR. Hemochromatosis: Discovery of the HFE Gene. Mo Med. 2012; 109(2): 133-136. PMID: 22675794.
Bardou-Jacquet E, Lainé F, Guggenbuhl P, Morcet J, Jézéquel C, Guyader D, Moirand R, Deugnier Y. Worse Outcomes of Patients With HFE Hemochromatosis With Persistent Increases in Transferrin Saturation During Maintenance Therapy. Clin Gastroenterol Hepatol. 2017;15(10):1620-1627. Doi: 10.1016/j.cgh.2016.12.039.
Beaton MD, Adam PC. The myths and realities of hemochromatosis. Can J Gastroenterol. 2007; 21(2):101-104. Doi: 10.1155/2007/619401.
Cassidy LM, Martiniano R, Murphy EM, Teasdale MD, Mallory J, Hartwell B, Bradley DG. PNAS. 2016;113(2):368-373. Doi: https://doi.org/10.1073/pnas.1518445113.
Distante S, Robson KJ, Graham-Campbell J, Arnaiz-Villena A, Brissot P, Worwood M. The origin and spread of the HFE-C282Y haemochromatosis mutation. Hum Genet. 2004;115(4):269-79. Doi: 10.1007/s00439-004-1152-4.
General Practice Supervisors Australia Authors. Hereditary Haemochromatosis. General Practice Supervisors Australia website. http://gpsupervisorsaustralia.org.au/teaching-plans/. Accessed March 25, 2020.
Haemochromatosis Australia Authors. Resources for GPs and Health Professionals. Haemochromatosis Australia website. https://haemochromatosis.org.au/gpresources/. Accessed March 25, 2020.
Hemochromatosis Org Authors. Hemochromatosis. Hemochromatosis Org website. https://www.hemochromatosis.org/#overview. Updated 2020. Accessed March 25, 2020.
Iron Disorder Institute Authors. Hemochromatosis Diagnosis Algorithm Clinical Evaluation & Management Protocol. Iron Disorder Institute website. http://www.irondisorders.org/Websites/idi/files/Content/854256/HHC%20ALL2011.pdf. Updated 2011. Accessed March 25, 2020.
John Hopkins Medicine Authors. Hemochromatosis. John Hopkins Medicine Gastroenterology & Hepatology website. https://www.hopkinsmedicine.org/gastroenterology_hepatology/_pdfs/liver/hemochromatosis.pdf. Accessed March 25, 2020.
Leah Rosenbaum & Marsha Morgan. Genetic haemochromatosis and sexual health in men. Trends in Urology & Men's Health, Online Library. https://onlinelibrary.wiley.com/doi/pdf/10.1002/tre.312. Updated January/February, 2013. Accessed March 26, 2020.
Mayo Clinic Authors. Hemochromatosis. Mayo Clinic website. https://www.mayoclinic.org/diseases-conditions/hemochromatosis/diagnosis-treatment/drc-20351448. Accessed March 27, 2020.
Royal Australian College of General Practitioners (RACGP) Authors. Genomics in general practice: Hereditary haemochromatosis. Royal Australian College of General Practitioners (RACGP) website. https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/genomics-in-general-practice/hereditary-haemochromatosis. Updated 2020. Accessed March 26, 2020.
Rune Johan Ulvik. Hereditary haemochromatosis through 150 years. Tidsskrift Den Norske Legeforening website. https://tidsskriftet.no/en/2016/12/medical-history/hereditary-haemochromatosis-through-150-years. Updated December 20, 2016. Accessed March 25, 2020.
Sebastiani G, Wilkinson N, K Pantopoulos. Pharmacological Targeting of the Hepcidin/Ferroportin Axis. Frontiers in Pharmacology. 206;7(316). doi: 10.3389/fphar.2016.00160
Thorm Milman N, Vinholt Schioedt F, Ellekaer Junker A, Magnussenc K. Diagnosis and Treatment of Genetic HFE-Hemochromatosis: The Danish Aspect. Gastroenterology Res. 2019;12(5):221–232. Doi: 10.14740/gr1206.
FIGURES
Figure 2. Biology Dictionary. Missense Mutation. Biology Dictionary website. https://biologydictionary.net/missense-mutation/. Accessed February 5, 2020.
ADDITIONAL SOURCE OF INFORMATION:
Annex 4 - Amino acids, one and three-letter codes.
Fact Sheet 47 | HEREDITARY HAEMOCHROMATOSIS.
Haemochromatosis, 3rd Edition, Digestive Health Foundation 2007.
Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. https://www.ncbi.nlm.nih.gov/books/NBK222309/.